CREW02 and ECHO Participating Sites
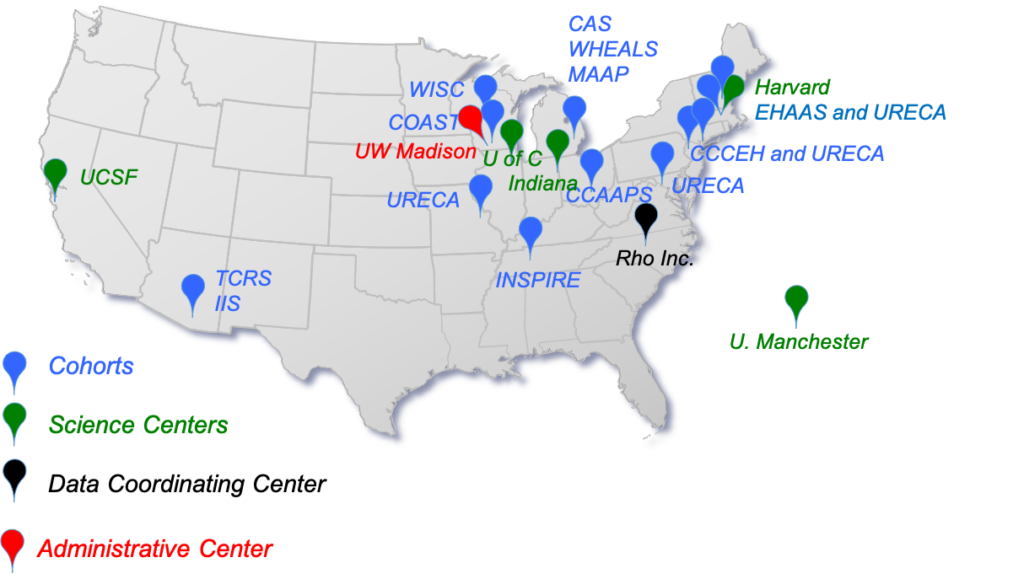
The Childhood Allergy/Asthma Study (CAS) – Henry Ford Health System, Detroit, MI
Initiated by Dennis Ownby and also led by Christine Johnson and Edward Zoratti (Henry Ford Health System, Detroit), begun in 1987, is a population-based study of general-risk children (n=835) in a suburban area north of Detroit. The main goal of the study was to identify early life exposures, including allergens, pets, and ETS, which influence asthma development. Samples collected include cord blood, perinatal medical record abstraction, home characteristics and exposures, and home dust, air sampling, urine and child and parental peripheral blood samples. Clinical information included pulmonary function, methacholine challenge, exhaled nitric oxide (eNO), IgE measurement and allergy skin tests. DNA and urine samples have been banked.
Contact Information
Principal Investigator: Dennis Ownby, MD
Lead Coordinator: Kyra Jones
Phone: 313-874-7390
Email: kjones20@hfhs.org
Studies
The Wayne County Health Environment Allergy and Asthma Longitudinal Study (WHEALS) – Henry Ford Health System, Detroit MI
Study led by Christine Johnson (Henry Ford Health System, Detroit MI), is a population-based birth cohort study being conducted in Detroit and Western Wayne County suburbs to examine the relationships between early life exposures such as pets, infections and the environmental and infant gut microbiome in relationship to the development of allergic diseases.
Contact Information
Principal Investigator: Christine Johnson, PhD, MPH
Lead Coordinator: Kyra Jones
Phone: 313-874-7390
Email: kjones20@hfhs.rog
Studies
The Microbes, Allergy, Asthma and Pets (MAAP)- Henry Ford Health System, Detroit MI
Study led by Edward Zoratti (Henry Ford Health System, Detroit MI) was established in 2014 and is ongoing as a birth cohort study designed to determine whether keeping dogs in the home influences early-life immune development, infants’ intestinal microbiota, and the risks of allergic diseases and asthma.
Contact Information
Principal Investigator: Edward Zoratti, MD
Lead Coordinator: Kyra Jones
Phone: 313-874-7390
Email: kjones20@hfhs.org
Studies
The Childhood Origins of Asthma (COAST) study – University of Wisconsin-Madison
Study led by Robert Lemanske Jr. and Daniel Jackson (University of Wisconsin-Madison), is a high-risk (parental history of asthma and/or allergy) birth cohort (n=289) that was established in 1998 to evaluate the contribution of both genetic and environmental (with emphasis on respiratory tract infections) factors in early life on the development of childhood asthma and allergic diseases.
Contact Information
Principal Investigator(s): Robert Lemanske Jr, MD & Daniel Jackson, MD
Phone: 608-228-9590 or 608-263-8539
Email: coast@medicine.wisc.edu or crew@medicine.wisc.edu
Studies
The Wisconsin Infant Study Cohort (WISC) – Marshfield Clinical Research Institute, Marshfield WI
Study led by James Gern (U. Wisconsin-Madison) is a birth cohort study conducted in rural Wisconsin (Marshfield area), and will include 100 infants born into dairy farm families and an equal number born into rural non-farm families. The study began enrollment in 2014, and recruitment is 85% complete. The goals of the study are to identify prenatal and early life exposures that affect immune development and respiratory illnesses in infancy.
Contact Information
Principal Investigator: Casper Bendixsen, PhD
Phone: 1-888-512-5488
Email: wiscstudy@marshfieldresearch.org
Website: https://www.marshfieldresearch.org/nfmc/wisc
Studies
The Cincinnati Childhood Allergy and Air Pollution Study (CCAAPS) – University of Cincinnati, OH
Study led by Patrick Ryan and Neeru Hershey (University of Cincinnati),is a longitudinal cohort of children (n=762) born to atopic parents in greater Cincinnati, OH. The objective of CCAAPS is to determine if exposure to traffic-related air pollution (TRAP), specifically diesel exhaust particles (DEP), is associated with the development of allergic disease and asthma and if this association is modified in a genetically at-risk population.
Contact Information
Principal Investigator(s): Patrick Ryan, PhD, MS & Gurjit (Neeru) Khurana Hershey,MD, PhD
Primary study contact: Liza Murrison
Phone: (513) 636-9041
Email: liza.murrison@cchmc.org
Studies
The Columbia Center for Children’s Environmental Health Cohort (CCCEH) – Columbia University, NYC
Study led by Frederica Perera and Rachel Miller (Columbia University, NYC), was established in 1998 and is a birth cohort of participants (n=727) living in Northern Manhattan and the South Bronx of NYC. The main goals of the CCCEH cohort are to undertake a comprehensive community-based assessment of environmental risks to African American and Latino infants and children living in Manhattan, and to develop strategies for reducing and ultimately preventing those risks.
Contact Information
Principal Investigator: Dr. Rachel Miller, MD
Study Coordinator: Judyth Ramirez-Carvey
Phone: (917) 891-0992
Email: jr379@cumc.columbia.edu
CCCEH study website: ccceh.org
Studies
The Epidemiology of Home Allergens and Asthma Study (EHAAS) – Harvard University, Boston MA
This study, established in 1994 and led by Diane Gold (Harvard University, Boston MA), is a high-risk (parental asthma and/or allergy) birth cohort (n=505) from Greater Boston that was established in 1994. EHAAS evaluates influences of early-life indoor home exposures to allergens, fungi, and microbial components on the development of wheeze, asthma, lung function, and related immune responses (e.g., allergic sensitization, IgE and IgG, innate or adaptive cytokine production, eNO). EHAAS has longitudinally evaluated parental and child perceived stress, life-events, socioeconomic stressors, and their associations with wheeze and immune responses.
Contact Information
Principal Investigator: Diane Gold, MD, MPH
Project Manager: Sharon O’Toole
Phone: 617-306-8855
Studies
The Urban Environment and Childhood Asthma (URECA) – Baltimore, Boston, St. Louis, New York City
Study led by James Gern (University of Wisconsin-Madison) is a high-risk birth cohort study (n=609) that was started in 2004 at four urban centers selected for high rates of poverty and asthma morbidity (Baltimore, Boston, St. Louis and New York City; Inner City Asthma Consortium). The goals of the study are to identify early life environmental factors in urban settings that affect immune development, wheezing illnesses, and asthma.
Contact Information
Site: Columbia University
Principal Investigator: Meyer Kattan, MD
Lead Coordinator: Perri Yaniv
Office number: (212) 305-6270
Site: Washington University
Principal Investigator: Katherine Rivera-Spoljaric, MD
Lead Coordinator: Beth Tesson
Email :tesson.elizabeth@wustl.edu
Site: Johns Hopkins University
Principal Investigator: Robert Wood, MD
Lead Coordinator: Paul Jones
Email :pjones48@jhmi.edu
Site: Boston University
Principal Investigator: George O’Connor
Lead Coordinator: Nicole Gonzalez<
Email :nicolegg@bu.edu
The Infant Susceptibility to Pulmonary Infections and Asthma Following RSV Exposure (INSPIRE) – Vanderbilt University, Nashville TN
Study, led by Tina Hartert, MD, MPH (Vanderbilt University, Nashville TN), is a prospective population-based non-selected birth cohort of 1,952 infants enrolled in the first few months of life between 2012-2014. INSPIRE study families are located throughout nine counties in Tennessee representing suburban, urban and rural areas of the state. The goals of the study are to identify and understand the effects of early life respiratory illnesses and microbial colonization on the subsequent development of childhood asthma. Environmental assessments in early life include viral diagnostics, pets, stress and behavior, household characteristics, LPS, personal microbiome, and dietary information.
Contact Information
Principal Investigator: Tina Hartert, MD, MPH
Phone: 615-875-BABY (2229) and 1-888-664-0505
Email: INSPIRE@vumc.org
Website: https://my.vanderbilt.edu/inspire/echo-crew/
Studies
CANOE Participating Sites
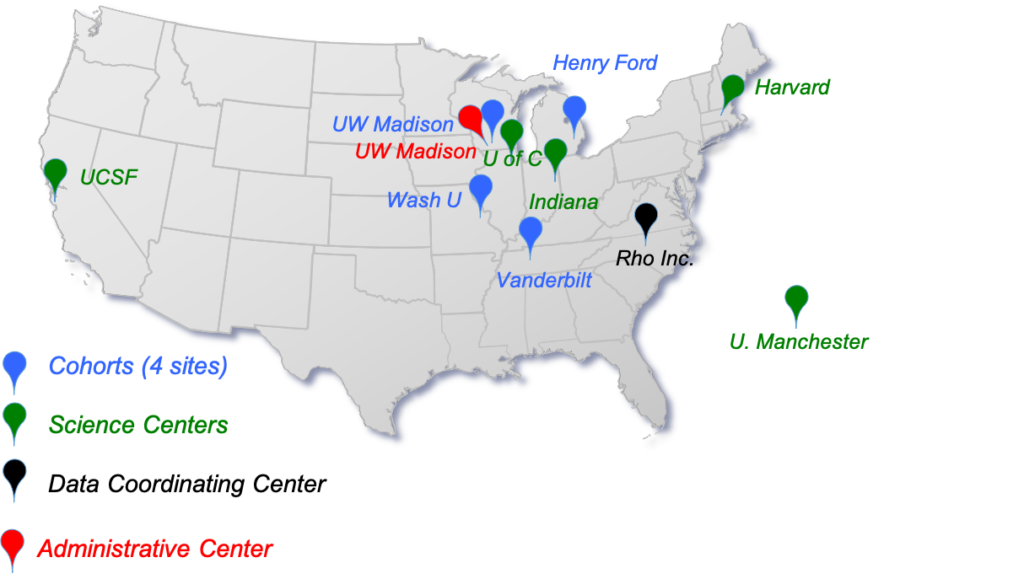
University of Wisconsin-Madison, Madison, WI
Principal Investigator: Anne Marie Singh, MD, Jim Gern, MDPrimary Study Contact(s): Jen Smith, Lisa Salazar, Mollie Schrodi, Jessica Fassbender
Lab number: 608-263-8539 or Study cell: 608-228-9590
Email: canoe@medicine.wisc.edu
Website: https://www.medicine.wisc.edu/asthma/canoe
Henry Ford Health System, Detroit, MI
Principal Investigator: Edward Zoratti, MD, Christine Joseph, PhDStudy contact information:
Phone: 888-883-8483
Email: CANOE@hfhs.org
Website: www.henryford.com/canoe
Washington University, St Louis, MO
Principal Investigator: Katherine Rivera-Spoljaric, MDPrimary study contact: Sam Williams and Beth Tesson
Phone: 314-286-1241
Email: swilliams32@wustl.edu and tesson.elizabeth@wustl.edu
Website: View Study
Contact information
Vanderbilt University Medical Center, Nashville, TN
Principal Investigator: Tina Hartert, MD/MPHLead Coordinator: Wais Folad, RN
Study contact information:
Phone: 615-322-4444
Email: canoe@vumc.org
Website: in progress**